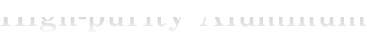HOME / High-purity aluminum manufacturing process
High-purity aluminum manufacturing process
Electrolytic smelting
As for the industrial process of manufacturing aluminum, there is a process that combines the Bayer process and the Hall-Heroult process. In the Bayer process, the alumina component in bauxite is dissolved and extracted in caustic soda to obtain alumina. Then, in the Hall-Heroult process, the alumina is dissolved in an electrolytic bath and electrolyzed to obtain aluminum. This process is called primary electrolytic smelting, and the purity of the obtained aluminum is about 99.7% to 99.98%. The melting point of alumina is just over 2000°C, but the operating temperature of the Hall-Heroult process is about 1000°C, which is below the melting point. It was the electrolytic bath used for electrolysis that made this process possible and the bath was discovered about 120 years ago by Mr. Hall and Mr. Heroult at about the same time. Since this bath can dissolve alumina in the same way that water dissolves sugar, it became possible to lower the operating temperature from a high temperature of over 2000°C to 1000°C, and the industrialization of aluminum manufacturing had progressed at one time.
Electrolytic refining furnace
Purification (Process for higher purification)
Two processes are industrially used to obtain even higher purity aluminum. One is the three-layer electrolytic process, and the other is the fractional crystallization process.
Three-layer electrolytic process
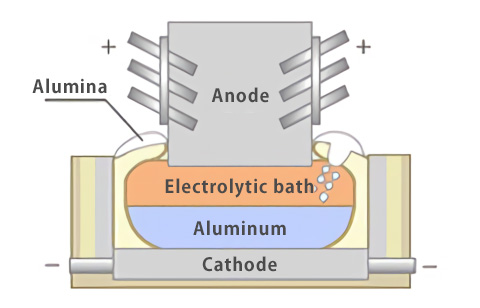
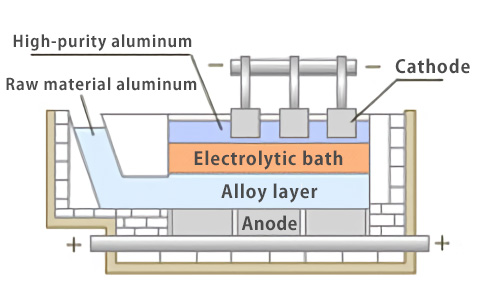
The three-layer electrolytic process is similar to the Hall-Heroult process described above and uses electric power. By inserting primary electrolytic aluminum, which is used as the raw material, into the alloy layer containing Cu and flowing electricity, only aluminum is collected on the cathode side. The purity of aluminum obtained by this process is about 99.98% to 99.998%.
Three-layer electrolytic process
Fractional crystallization equipment
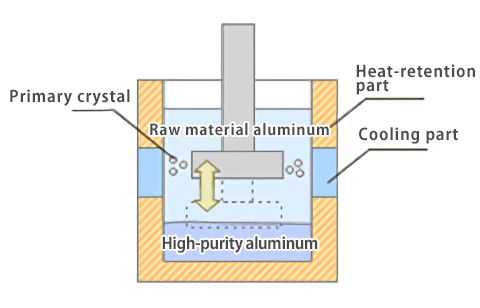
In the fractional crystallization process, refinement is performed by a mechanism different from the Hall-Heroult process and the three-layer electrolytic process. Unlike the two processes described above that use electrochemical processes, the fractional crystallization process uses a thermodynamic method to obtain high-purity aluminum. The fractional crystallization method uses a principle that, when the primary electrolytic aluminum used as the raw material is melted and cooled locally, high-purity aluminum crystallizes as primary crystal. The melted primary electrolytic aluminum is locally cooled, and the primary crystal crystallized is separated. The purity of aluminum obtained by this process is about 99.98% to 99.996%.
Fractional crystallization equipment